- Interactive map visualizes care deserts in U.S.: Up to 60 million Americans in rural areas face limited access to qualified treatment centers
- 99% of surveyed oncologists agree that cell and gene therapies are among the most important innovations of our time, but cite hurdles ahead
- Report developed by InspiroGene by McKesson, a dedicated business to support the commercialization of cell and gene therapies
InspiroGene™ by McKesson, a dedicated business focused solely on supporting the commercialization of cell and gene therapies (CGTs), today announced the publication of its inaugural 2024 Cell and Gene Therapy Report: Advancing the Future of Medicine.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241009596023/en/
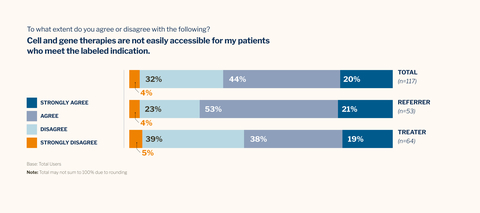
As part of the 2024 Cell and Gene Therapy Report, McKesson worked with a third party market research firm to conduct a double-blind online survey of 124 U.S. medical oncologists and hematological oncologists in June and July 2024. “Total users” refers to the 117 participants with direct experience treating patients with cell and gene therapies (CGTs) or referring patients for CGTs. (Graphic: Business Wire)
Designed to deliver unique insights about the CGT landscape and its future trajectory, the report includes an overview of the U.S. CGT pipeline, findings from a survey of 124 U.S. oncologists capturing their perspectives on these therapies, a newly developed interactive map of qualified CGT treatment centers nationwide, and interviews with industry experts.
“With more than 30 FDA approved cell and gene therapies available in the U.S., the potential to deliver transformative care, or even cures, to patients with serious illnesses has never been greater,” said Joe DePinto, Head of Cell, Gene, and Advanced Therapies, McKesson. “Despite the compelling science, significant barriers prevent many patients from obtaining these life-changing therapies. Our 2024 report delves into these challenges and proposes possible solutions to broaden access for more patients.”
To better understand the current use of CGTs and possible barriers to future adoption, InspiroGene conducted a survey of 124 oncologists. The survey aimed to gather insights on how, when, and why these specialists prescribe these therapies to their patients. Key findings from the report include:
- 99% of oncologists agree that CGTs are among the most important medical innovations of our time. However, 64% also agree that therapies are not easily accessible for patients who meet the labeled indications.
- Three out of five physicians say patients they refer for CGTs often receive other treatments instead. When asked, they cite insurance coverage and out-of-pocket costs as the most common reasons.
- 47% of oncologists say their patients are “rarely” or “never” aware that CGTs are available for their condition.
The report also explores challenges to equitable access to CGTs, including the concentration of delivery of care at academic medical centers in major metropolitan areas, leaving many Americans with little to no access. McKesson’s research into qualified treatment centers across the U.S., depicted in the report and in an interactive map, illustrates that “CGT deserts” exist across the nation. The report highlights the barriers to advanced care faced by patients and their providers in these underserved areas and examines strategies for transitioning treatment into community settings.
Other features of the report include:
- Pipeline data illustrating development of CGT in broader therapeutic areas, beyond oncology, such as cardiovascular disease, diabetes, and central nervous system disorders.
- Expert perspectives by leaders from medical centers, research centers, community practices, and industry think tanks, exploring barriers to CGT adoption and potential solutions.
The full 2024 Cell and Gene Therapy Report can be found at www.inspirogene.com/2024cgtreport.
About InspiroGene by McKesson
InspiroGene by McKesson (“InspiroGene”) is a dedicated business unit focused solely on supporting the commercialization of cell and gene therapies (CGTs). At InspiroGene, we turn CGT innovation into a reality. We offer flexible, sustainable solutions to help manufacturers, payers, and providers navigate the complex CGT commercialization landscape. As an enduring ally, we’re dedicated to transforming patient care and driving better health outcomes. Learn more about the InspiroGene advantage at InspiroGene.com.
About McKesson Corporation
McKesson Corporation is a diversified healthcare services leader dedicated to advancing health outcomes for patients everywhere. Our teams partner with biopharma companies, care providers, pharmacies, manufacturers, governments, and others to deliver insights, products, and services to help make quality care more accessible and affordable. Learn more about how McKesson is impacting virtually every aspect of healthcare at McKesson.com and read Our Stories.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241009596023/en/
Contacts
PR Contact
InspiroGene by McKesson
Lindsay Yanek, Communications
Lindsay.Yanek@McKesson.com













