Ozempic usage for weight loss has gone viral, making it the highest revenue-generating drug for Novo Nordisk AV/S (NYSE: NVO). Originally purposed for Type 2 diabetes, the skyrocketing off-label use for weight loss led Novo to create Wegovy, a stronger version of Ozempic (generic name: semaglutide) for obesity.
There seems to be a GLP-1 gold rush in the medical sector. Novo competitor Ely Lilly & Co. (NYSE: LLY) is experiencing such unprecedented demand for its GLP-1/GIP dual agonist drugs Mounjaro and Zepbound that they're constructing a $2.5 billion manufacturing plant to handle the demand. Both drugs are breaking records, and the demand has made it difficult to keep them stocked.
Ozempic, Mounjaro and Zepbound are all used to manage Type 2 diabetes but differ in their mechanisms and manufacturers. This competition drives innovation and gives patients options for diabetes management.
Expanding the Indications
Due to its costs, many health insurers and employers have restricted reimbursements for using Ozempic for weight-loss purposes. Novo is taking steps to expand the coverage for Wegovy to include more indications in areas of chronic disease treatment, including obesity-related pulmonary and cardiovascular diseases, and potentially neurological disorders.
Semaglutide for Cardiovascular Disease
Novo Nordisk released a study indicating improved symptoms for patients with obesity, diabetes and heart failure. The study called "Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes" was published in the New England Journal of Medicine and presented at the American College of Cardiology conference. Novo completed two trials and wants to attain a new indication of heart failure with preserved ejection fraction (HFpEF).
Ejection fraction is a measure of how the left ventricle, which is the main pumping chamber, can squeeze with every beat. Normal ejection fracture should be above 50%. However, preserved ejection fracture (HFpEF) tends to be above 50%, but the function of the heart is still impaired.
What the Study Entails
Novo states that there are no approved therapies that target obesity-related heart failure with preserved ejection fraction in persons with Type 2 diabetes. This indicates a need.
The study of 616 participants was comprised of random patients who have had heart failure with preserved ejection fraction and a bodyweight mass index (BMI) above 30 with Type 2 diabetes. Each patient received a weekly 2.4 mg or a placebo dose for 52 weeks.
The primary endpoint was the change in their Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ-CSS). The scores range from 0 to 100, with higher scores indicating fewer symptoms phy, physical limitations and body weight change. Secondary endpoints were changes in the six-minute walk distance, heart failure events and hierarchical composite endpoints.
The Study Results
Of the 616 participants, those who took the semaglutide had superior improvements in weight loss (9.8% versus 3.4% in the placebo) and heart-related quality of life than placebo participants (13.7 points versus 6.4 points with placebo). Semaglutide also improved inflammation and walking distances. Surprisingly, serious adverse events were reported by 55 participants or 17.7%, in the semaglutide group versus 88 participants or 28.8%, in the placebo group.
The Conclusion
The study concluded that patients with obesity-related heart failure (HFpEF) and Type 2 diabetes who took semaglutide experienced larger reductions in heart failure-related symptoms, greater weight loss and physical limitations than the placebo in one year.
GLP-1 and Parkinson's Disease
Barclay noted a recent study that GLP-1 agonists appeared to benefit patients with Parkinson's Disease. A 156-patient study was performed with patients using a GLP-1 medication by Sanofi (NASDAQ: SNY) called lixisenatide. The participants taking lixisenatide experienced no worsening of motor disabilities at the one-year mark, indicating the effects of Parkinson's were slowed down.
Novo Nordisk is also exploring the effects of GLP-1 on Alzheimer's Disease. The potential for off-label use could be a boon to the company.
Novo Nordisk analyst ratings and price targets are at MarketBeat.
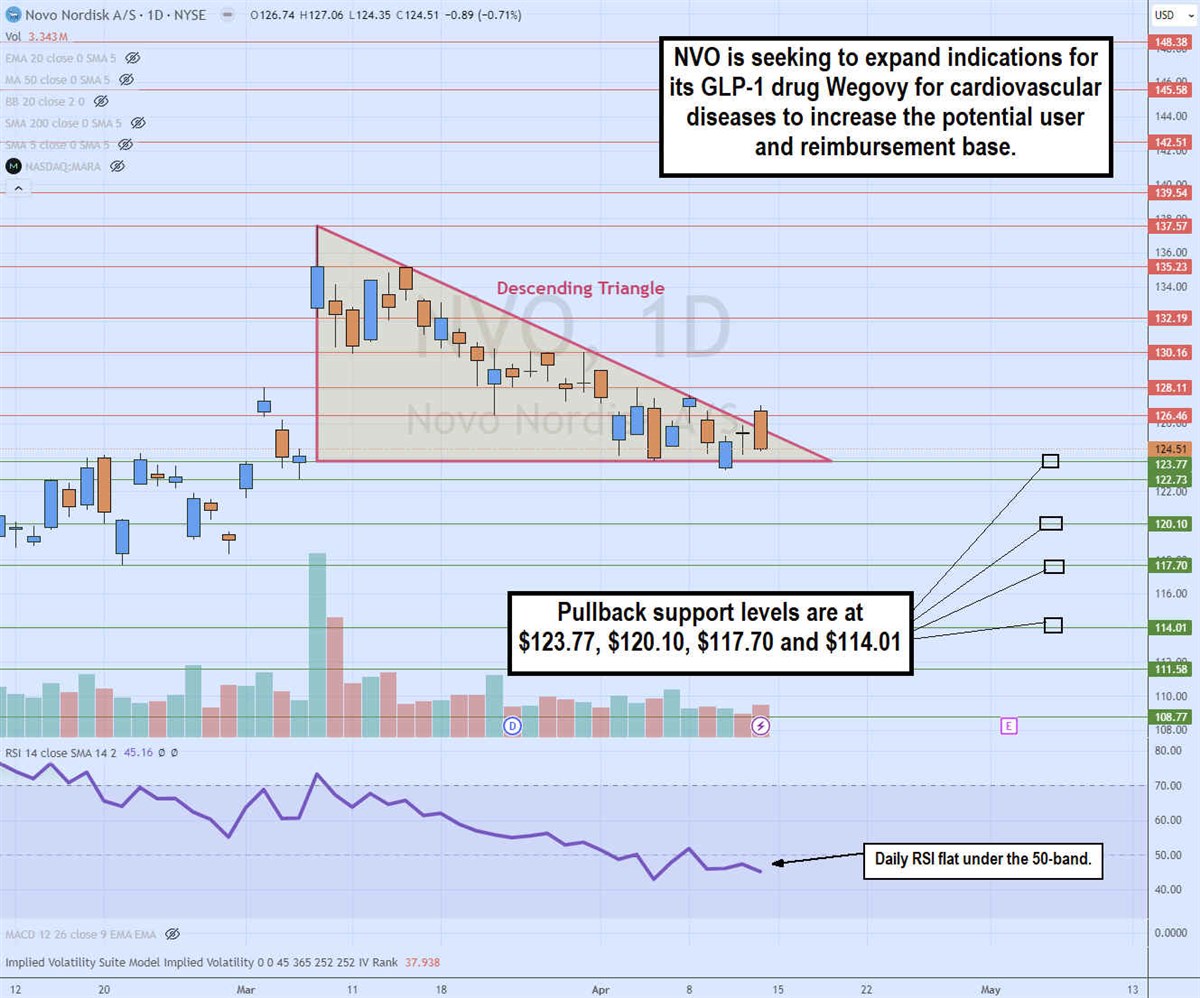
Daily Descending Triangle Pattern
The daily candlestick chart on NVO illustrates a descending triangle pattern. The descending trendline formed at the $137.57 swing high, capping bounces at lower highs. The flat-bottom lower trendline is at $123.77. NVO is trading close to the apex point indicating an imminent breakout through the upper trendline or breakdown through the flat-bottom trendline will develop. The daily relative strength index (RSI) is chopping flatly around the 50-band. Pullback support levels are at $123.77, $120.10, $117.70 and $114.01.














